 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| EP-155 | Human | Integrin α 4 β 7 : MAdCAM-1 [Biotinylated] Inhibitor Screening ELISA Kit | |||
| IT7-H52W4 | Human | Human Integrin alpha 4 beta 7 (ITGA4&ITGB7) Heterodimer Protein, His Tag&Tag Free (MALS & SPR verified) | 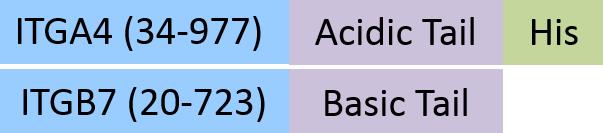 |

|
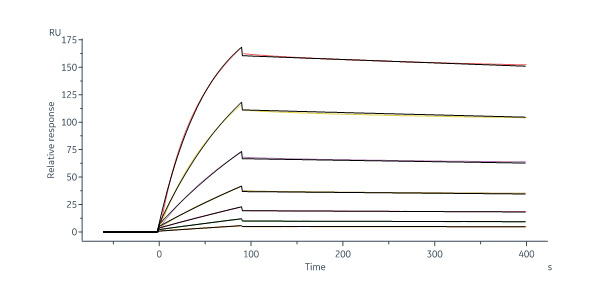
Anti-Human Integrin α 4 β 7 MAb, Human IgG1 (ADCC Disable) captured on Protein A Chip can bind Human ITGA4&ITGB7 Heterodimer Protein, His Tag&Tag Free (IT7-H52W4) with an affinity constant of 1.37 nM as determined in a SPR assay (Biacore 8K) (QC tested).
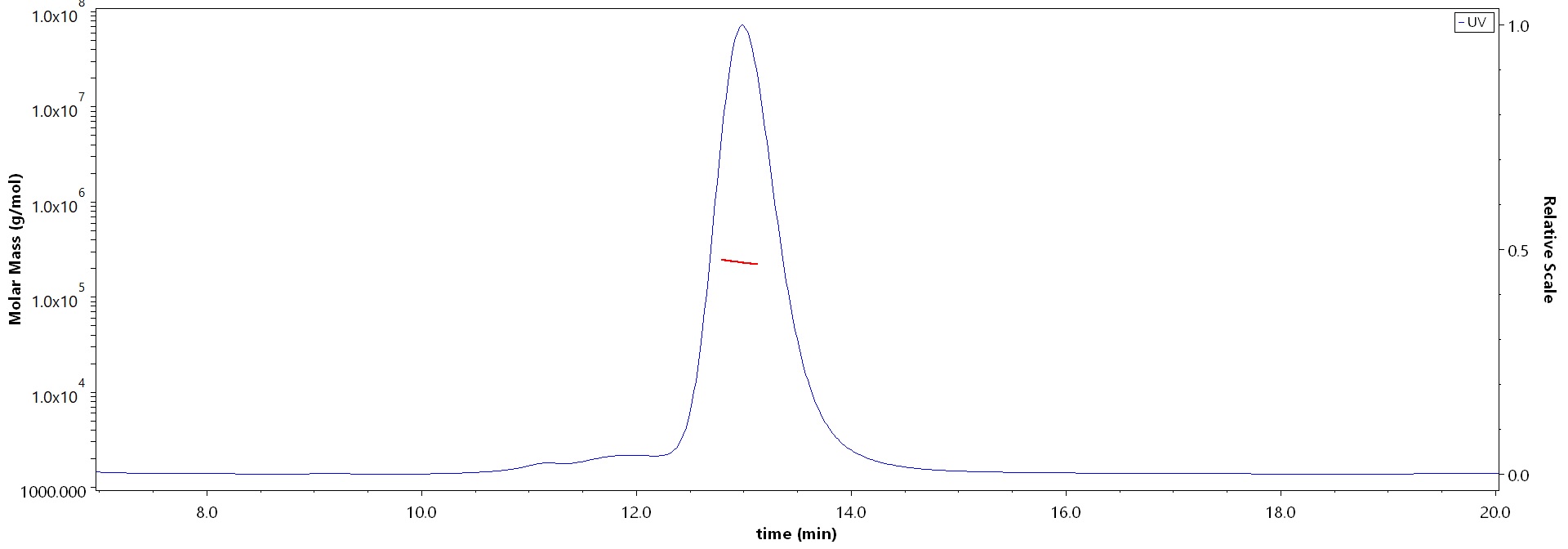
The purity of Human ITGA4&ITGB7 Heterodimer Protein, His Tag&Tag Free (Cat. No. IT7-H52W4) is more than 85% and the molecular weight of this protein is around 210-250 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Carotegrast methyl | AJM-300 | Approved | Ea Pharma, Eisai Co Ltd | Carogra | Japan | Colitis, Ulcerative | Ea Pharma Co Ltd | 2022-03-28 | Colitis, Ulcerative | Details |
| Vedolizumab | MLN-0002; LPD-02; MLN-002; MLN-02; LDP-02 | Approved | Millennium Pharmaceuticals Inc | 安吉优, Entyvio | United States | Colitis, Ulcerative; Crohn Disease | Takeda Pharmaceuticals U.S.A. Inc | 2014-05-20 | Colitis; Melanoma; Carcinoma, Non-Small-Cell Lung; Crohn Disease; Urogenital Neoplasms; Colitis, Ulcerative; Pediatric ulcerative colitis; Celiac Disease; Cholangitis, Sclerosing; Solid tumours; Neoplasms; Pouchitis; Pediatric Crohn's disease; Inflammatory Bowel Diseases; Acquired Immunodeficiency Syndrome; Hematopoietic stem cell transplantation (HSCT); HIV Infections | Details |
| Natalizumab biosimilar(Polpharma Biologics) | PB-006; DST-356A1 | Approved | Polpharma Biologics Sa | Tyruko | United States | Crohn Disease; Multiple Sclerosis | Sandoz Inc | 2023-08-24 | Multiple Sclerosis, Relapsing-Remitting; Multiple Sclerosis; Crohn Disease | Details |
| Natalizumab | BG-0002; TY-21.6; AN-10022; BG-00002; AN-100226; BG-0002-E | Approved | Biogen Inc, Perrigo Llc | Tysabri, Antegran, Antegren | United States | Multiple Sclerosis; Crohn Disease | Biogen Idec | 2004-11-23 | Multiple Sclerosis, Relapsing-Remitting; Epilepsies, Partial; Arthritis, Rheumatoid; Graft vs Host Disease; Stroke; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Multiple Myeloma; Demyelinating Diseases; Myositis, Inclusion Body; Crohn Disease | Details |
| Carotegrast methyl | AJM-300 | Approved | Ea Pharma, Eisai Co Ltd | Carogra | Japan | Colitis, Ulcerative | Ea Pharma Co Ltd | 2022-03-28 | Colitis, Ulcerative | Details |
| Vedolizumab | MLN-0002; LPD-02; MLN-002; MLN-02; LDP-02 | Approved | Millennium Pharmaceuticals Inc | 安吉优, Entyvio | United States | Colitis, Ulcerative; Crohn Disease | Takeda Pharmaceuticals U.S.A. Inc | 2014-05-20 | Colitis; Melanoma; Carcinoma, Non-Small-Cell Lung; Crohn Disease; Urogenital Neoplasms; Colitis, Ulcerative; Pediatric ulcerative colitis; Celiac Disease; Cholangitis, Sclerosing; Solid tumours; Neoplasms; Pouchitis; Pediatric Crohn's disease; Inflammatory Bowel Diseases; Acquired Immunodeficiency Syndrome; Hematopoietic stem cell transplantation (HSCT); HIV Infections | Details |
| Natalizumab biosimilar(Polpharma Biologics) | PB-006; DST-356A1 | Approved | Polpharma Biologics Sa | Tyruko | United States | Crohn Disease; Multiple Sclerosis | Sandoz Inc | 2023-08-24 | Multiple Sclerosis, Relapsing-Remitting; Multiple Sclerosis; Crohn Disease | Details |
| Natalizumab | BG-0002; TY-21.6; AN-10022; BG-00002; AN-100226; BG-0002-E | Approved | Biogen Inc, Perrigo Llc | Tysabri, Antegran, Antegren | United States | Multiple Sclerosis; Crohn Disease | Biogen Idec | 2004-11-23 | Multiple Sclerosis, Relapsing-Remitting; Epilepsies, Partial; Arthritis, Rheumatoid; Graft vs Host Disease; Stroke; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Multiple Myeloma; Demyelinating Diseases; Myositis, Inclusion Body; Crohn Disease | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Etrolizumab | PRO-145223; rhuMAb-Beta7; R-7413; RG-7413 | Phase 3 Clinical | Genentech Inc | Colitis, Ulcerative; Crohn Disease | Details |
| Vedolizumab biosimilar (Polpharma Biologics) | PB-016 | Phase 3 Clinical | Polpharma Biologics Sa | Pouchitis; Colitis, Ulcerative; Crohn Disease | Details |
| MORF-057 | MORF-057 | Phase 2 Clinical | Morphic Therapeutic Inc | Inflammatory Bowel Diseases; Colitis, Ulcerative; Crohn Disease | Details |
| PN-10943 | PN-10943-A; PN-10943; PN-943 | Phase 2 Clinical | Protagonist Therapeutics Inc | Colitis, Ulcerative | Details |
| GS-1427 | GS-1427 | Phase 2 Clinical | Inflammatory Bowel Diseases; Colitis, Ulcerative | Details | |
| ABBV-382 | Phase 2 Clinical | Abbvie Inc | HIV Infections | Details | |
| PTG-100 | PTG-100 | Phase 1 Clinical | Protagonist Therapeutics Inc | Celiac Disease; Colitis, Ulcerative | Details |
| Etrolizumab | PRO-145223; rhuMAb-Beta7; R-7413; RG-7413 | Phase 3 Clinical | Genentech Inc | Colitis, Ulcerative; Crohn Disease | Details |
| Vedolizumab biosimilar (Polpharma Biologics) | PB-016 | Phase 3 Clinical | Polpharma Biologics Sa | Pouchitis; Colitis, Ulcerative; Crohn Disease | Details |
| MORF-057 | MORF-057 | Phase 2 Clinical | Morphic Therapeutic Inc | Inflammatory Bowel Diseases; Colitis, Ulcerative; Crohn Disease | Details |
| PN-10943 | PN-10943-A; PN-10943; PN-943 | Phase 2 Clinical | Protagonist Therapeutics Inc | Colitis, Ulcerative | Details |
| GS-1427 | GS-1427 | Phase 2 Clinical | Inflammatory Bowel Diseases; Colitis, Ulcerative | Details | |
| ABBV-382 | Phase 2 Clinical | Abbvie Inc | HIV Infections | Details | |
| PTG-100 | PTG-100 | Phase 1 Clinical | Protagonist Therapeutics Inc | Celiac Disease; Colitis, Ulcerative | Details |
This web search service is supported by Google Inc.
